how are covalent compounds named?
A non-metal non-metal pair. Second element is named as an Anion suffix -ide 3.

Covalent Molecules Eq How Are The Chemical Formulas And Chemical Names Written For Covalent Molecules Ppt Download
The first element in the formula is simply listed using the name of the element.

. They are named as multiplying prefixname of first element multiplying prefixname of second element two words If there is only one atom of the first element the multiplying prefix is omitted. The names of the two atoms are joined together to form the compound name. Rules for Binary Covalent Compounds. Valence electrons are the outermost electrons of an atom.
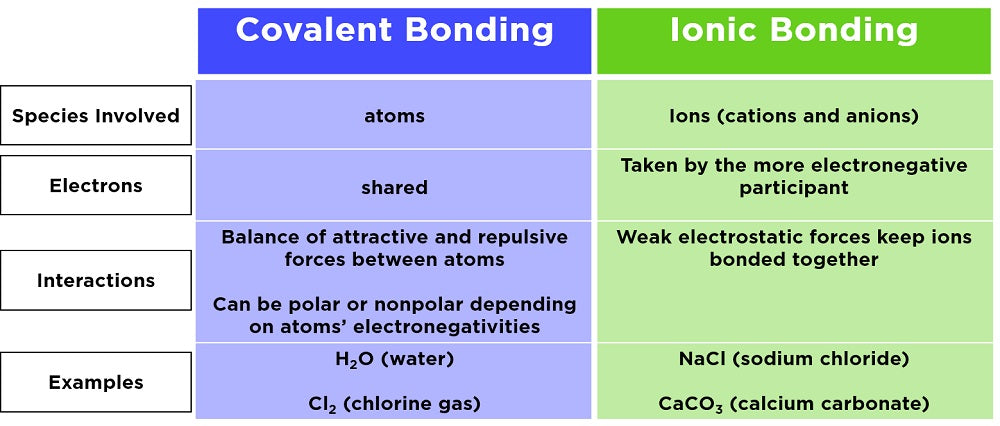
Covalent compounds are named after the atoms that form them. Covalent compounds are formed when two or more nonmetal atoms bond by sharing valence electrons. The second element is named by taking the stem of the element name and adding the suffix -ide. Make sure the compound youre trying to name is actually covalent.
If youre still reading go to step 2. Molecular compounds or covalent compounds are those in which the elements share electrons via covalent bonds. A system of numerical prefixes is used to specify the number of atoms in a molecule. Covalent compounds are formed when two nonmetal atoms create a covalent bond by sharing valence electrons.
Mono is not used to name the. This is a covalent compound made up of only two different elements. The prefix mono is never used for naming the first element of a compound. The only type of molecular compound a chemistry student is expected to be able to name is a binary covalent compound.
Remember its only the final o or aSo the name of ClO 2 will be chlorine dioxide and no vowels are dropped. Well theres one common way to name covalent compounds. The less electronegative element is named first. Binary covalent compounds contain only two elements.
Prefixes are used to denote the number of atoms 4. Naming covalent compounds involves the use of prefixes to identify the amount of each element inv. The final o or a of a prefix is often dropped when the element begins with a vowel. Unlike ionic compounds covalent molecules exist as true molecules.
If the compound youre naming has a metal atom or the ammonium ion somewhere in it its an ionic compound and youre in the wrong tutorial. If it is a H O F Br I N Cl then if it is a two atom molecule it would just be its name so O2 is oxygen. How are ionic compounds named. In this video we will learn how to name covalent compounds.
The first element is named first using the elements name. Updated January 27 2019. The less electronegative element is named first. Go back to the ionic naming tutorial and use those steps to solve the problem.
These elements will be found on the far right side of the periodic table -. The first element in. The ending of the second atoms name is changed in the same way as for negative ions. Naming binary two-element covalent compounds is similar to naming simple ionic compounds.
These compounds are formed when two nonmetals are held together by a covalent bond. Nonmetals are all very electronegative and form bonds through the sharing of electrons to satisfy the octet rule. If the molecule is more complex then you would use the prefix chart to put a prefix corresponding to the number of that atom. NAMING COVALENT COMPOUNDS.
Covalent compounds are compounds formed by two elements that are NONMETAL. Naming Covalent Compounds or Molecular Compounds When Given the Formula Covalent compounds often called molecular compounds consist of two non-metals. Naming covalent compounds Naming binary two-element covalent compounds is similar to naming simple ionic compounds. Because electrons are shared in covalent molecules no.
For instance NaCl is an ionic compound because sodium is a metal and chlorine is a nonmetal. Binary covalent compounds contain only two. For example for CO the name will be carbon monoxide and the final o of mono is dropped. Learn about the formation of covalent compounds the properties and naming of.
When you see a compound with two or more nonmetals. They are named as multiplying prefixname of first element multiplying prefixname of second element two words If there is only one atom of the first element the multiplying prefix is omitted. First of all to name a covalent compound it helps to know what a covalent compound is.

Covalent Bonding Eq How Are The Chemical Formulas And Chemical Names Written For Covalent Molecules How Do You Draw Vsepr Diagrams For Covalent Compounds Ppt Download

Covalent Bond Examples Formation Properties What Is A Covalent Bond Video Lesson Transcript Study Com

Covalent Compounds Covalent Bond Properties Examples With Videos

Covalent Structures Eq How Are The Chemical Formulas And Chemical Names Written For Covalent Molecules How Do You Draw Vsepr Diagrams For Covalent Compounds Ppt Download

Posting Komentar untuk "how are covalent compounds named?"